Complexity Thoughts: Issue #67
Unraveling complexity: building knowledge, one paper at a time
If you find value in #ComplexityThoughts, consider helping it grow by subscribing and sharing it with friends, colleagues or on social media. Your support makes a real difference.
→ Don’t miss the podcast version of this post: click on “Spotify/Apple Podcast” above!
Foundations of network science and complex systems
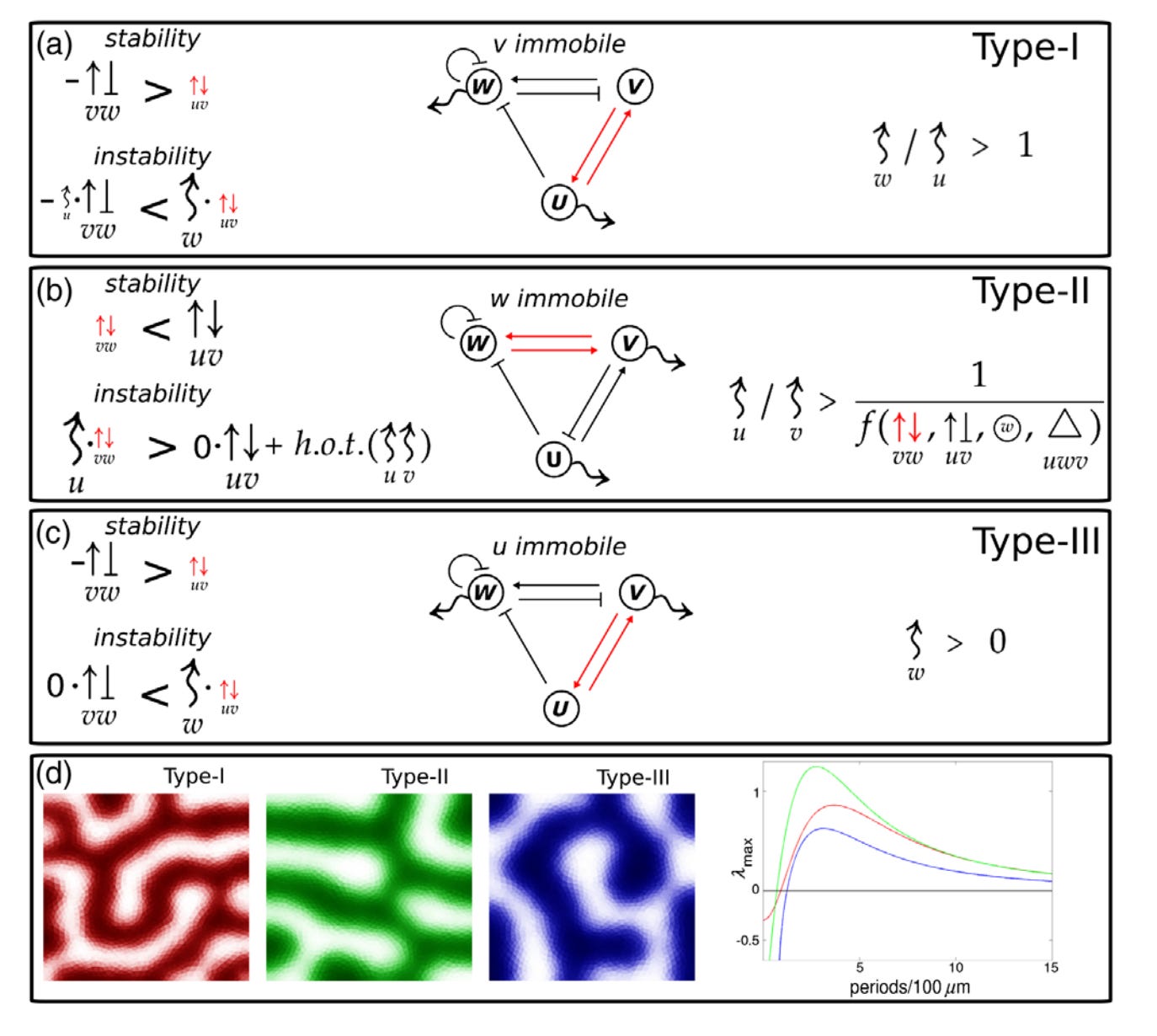
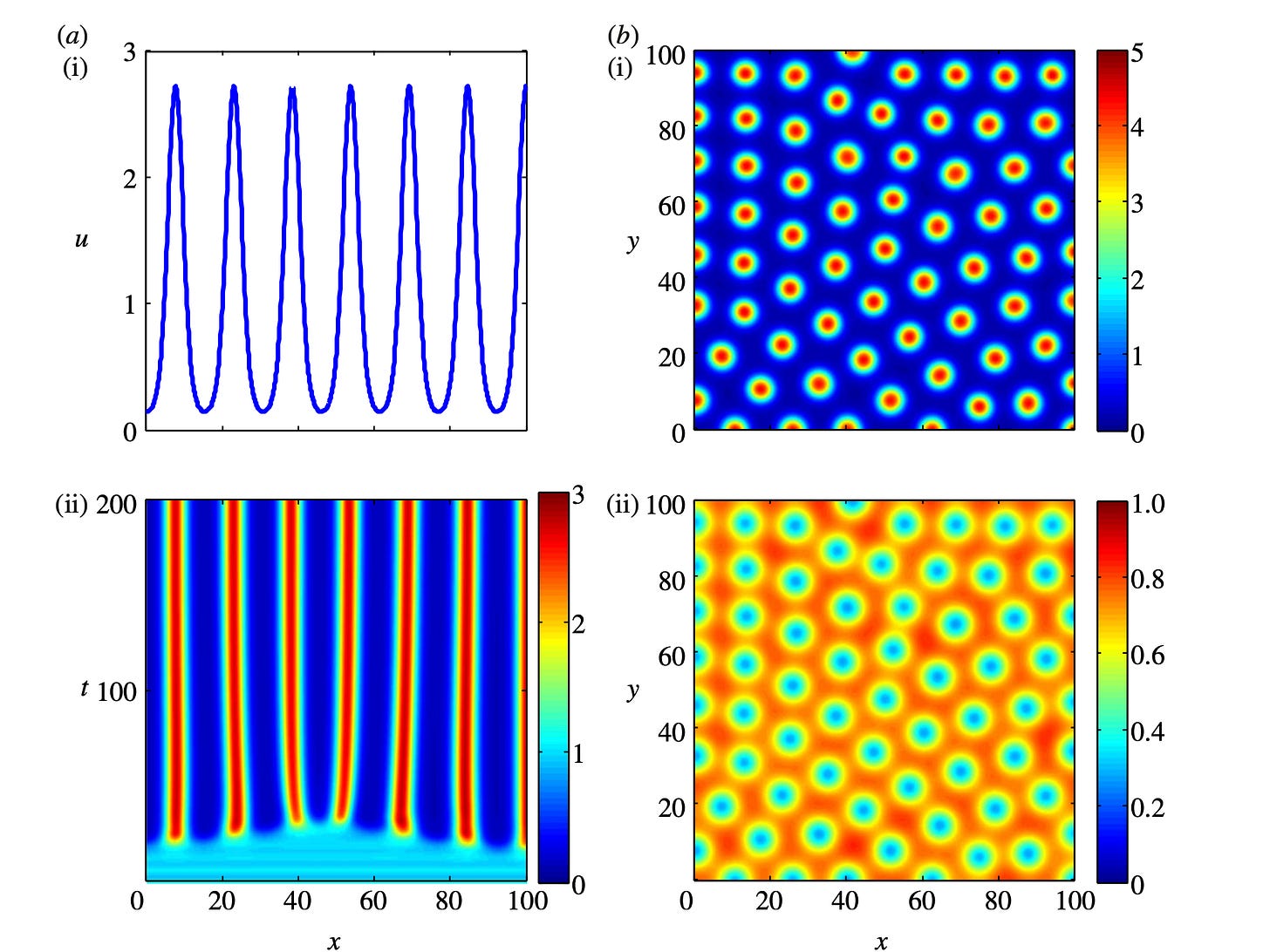
Key Features of Turing Systems are Determined Purely by Network Topology
In 1952, Alan Turing formulated a groundbreaking theory that explained how spatial organization emerged in embryonic tissues. This theory has since been proposed to explain the emergence of spatial organization in systems as diverse as chemical reactions, cardiac arrhythmias, semiconductors, and even urban criminal activity. However, the original theory imposes requirements that are rarely met in reality, casting its relevance into doubt. We present a new theory that explains how the limitations in Turing’s theory arise and how to eliminate them altogether.
In its original form, a Turing system consists of two molecular species that must diffuse at very different rates to produce a pattern. Such a difference is typically not found in real systems. Also, most Turing models require a level of fine-tuning that prevents them from being a robust mechanism for any real patterning process. Researchers have found partial solutions to these limitations but lack a definitive understanding of their source.
Our theory demonstrates how the topology of a Turing system determines these limitations. Further, we prove that an appropriate topological arrangement of the feedback between the system’s components can eliminate these limitations altogether. We also discover the rules that determine the spatial overlap of the different Turing species (e.g., the different molecules) in the pattern.
These findings should dispel objections made against the role of Turing patterns in biological development. Our results should help to design synthetic Turing systems and to engineer tissues with specific genes coexpressed.
Turing’s theory of pattern formation is a universal model for self-organization, applicable to many systems in physics, chemistry, and biology. Essential properties of a Turing system, such as the conditions for the existence of patterns and the mechanisms of pattern selection, are well understood in small networks. However, a general set of rules explaining how network topology determines fundamental system properties and constraints has not been found. Here we provide a first general theory of Turing network topology, which proves why three key features of a Turing system are directly determined by the topology: the type of restrictions that apply to the diffusion rates, the robustness of the system, and the phase relations of the molecular species.
Turing's model for biological pattern formation and the robustness problem
One of the fundamental questions in developmental biology is how the vast range of pattern and structure we observe in nature emerges from an almost uniformly homogeneous fertilized egg. In particular, the mechanisms by which biological systems maintain robustness, despite being subject to numerous sources of noise, are shrouded in mystery. Postulating plausible theoretical models of biological heterogeneity is not only difficult, but it is also further complicated by the problem of generating robustness, i.e. once we can generate a pattern, how do we ensure that this pattern is consistently reproducible in the face of perturbations to the domain, reaction time scale, boundary conditions and so forth. In this paper, not only do we review the basic properties of Turing's theory, we highlight the successes and pitfalls of using it as a model for biological systems, and discuss emerging developments in the area.
Evolution
Flourishing chemosynthetic life at the greatest depths of hadal trenches
Our findings also challenge the traditional perspective on the energy sources sustaining hadal fauna, which were predominantly believed to be derived from surface-derived particulate organic matter and carrion fall
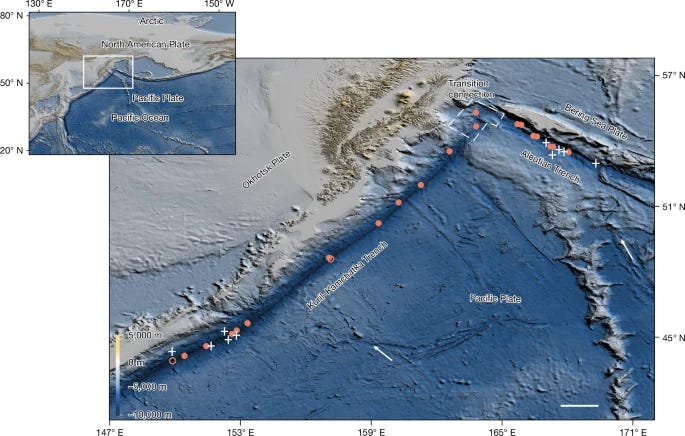
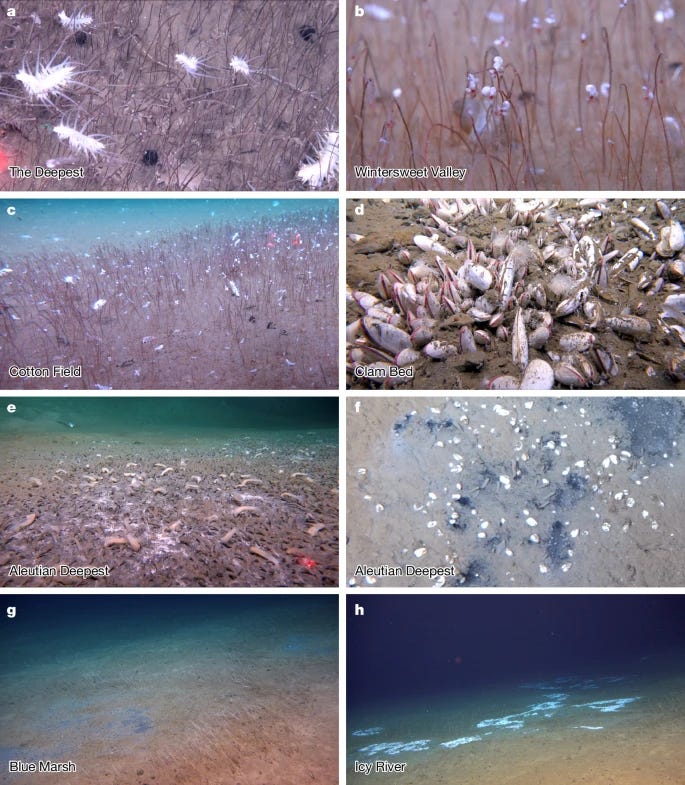
Hadal trenches, some of the Earth’s least explored and understood environments, have long been proposed to harbour chemosynthesis-based communities1,2. Despite increasing attention, actual documentation of such communities has been exceptionally rare3,4. Here we report the discovery of the deepest and the most extensive chemosynthesis-based communities known to exist on Earth during an expedition to the Kuril–Kamchatka Trench and the western Aleutian Trench using the manned submersible Fendouzhe. The communities dominated by siboglinid Polychaeta and Bivalvia span a distance of 2,500 km at depths from 5,800 m to 9,533 m. These communities are sustained by hydrogen sulfide-rich and methane-rich fluids that are transported along faults traversing deep sediment layers in trenches, where methane is produced microbially from deposited organic matter, as indicated by isotopic analysis. Given geological similarities with other hadal trenches, such chemosynthesis-based communities might be more widespread than previously anticipated. These findings challenge current models of life at extreme limits and carbon cycling in the deep ocean.
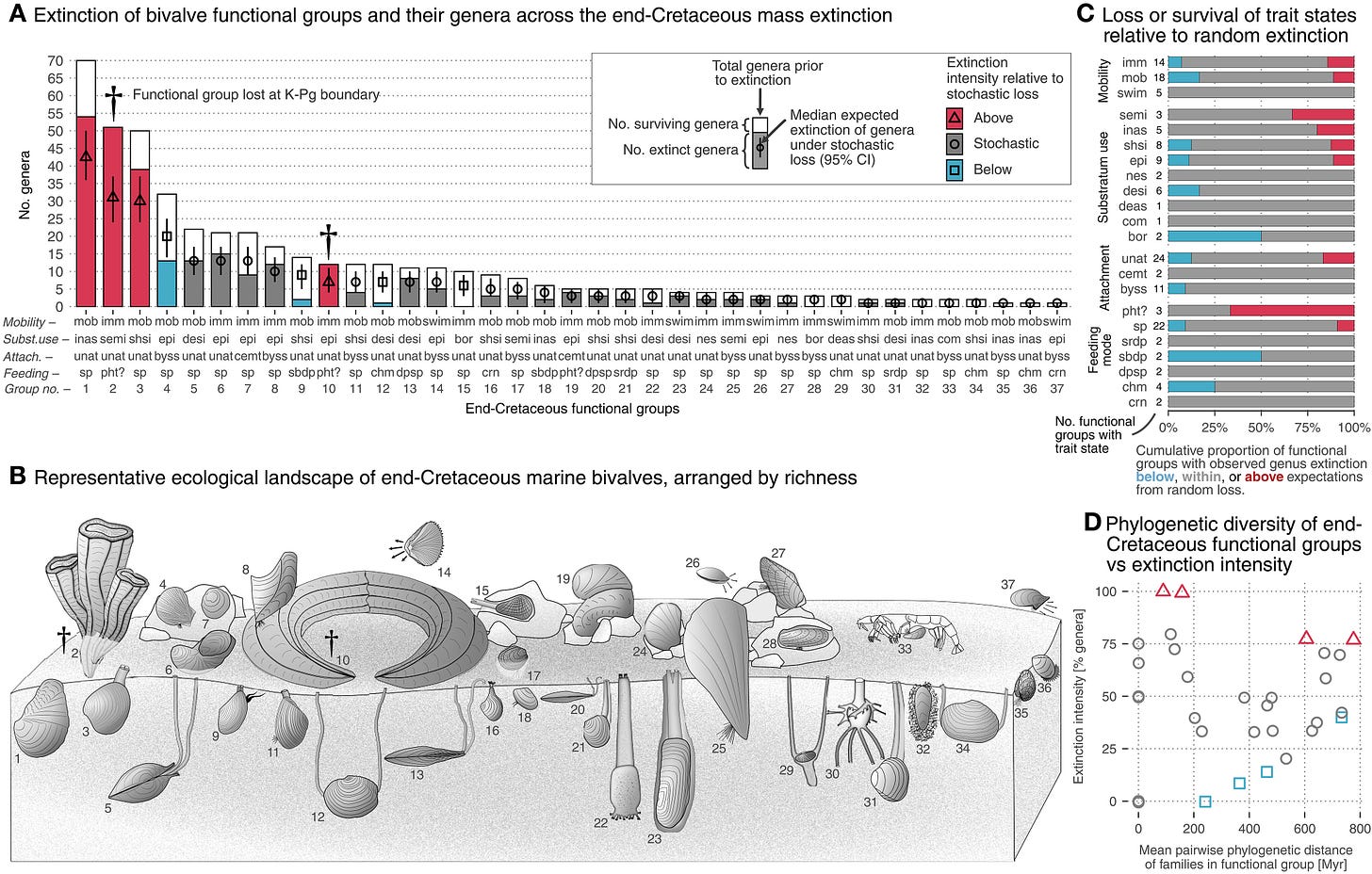
Sixty-six million years ago, the same asteroid that doomed the dinosaurs also wiped out most marine bivalve species. However, and also rather surprisingly, their ecosystems barely lost functional diversity. This paper shows that while biodiversity collapsed, the surviving species kept ecosystems running, though in a reshuffled way. I think that this is a striking reminder for today: mass extinctions may not instantly erase ecosystem function, but the (hidden) reorganization could shape how nature recovers in the long run.
The end-Cretaceous (K-Pg) mass extinction shows how large-scale taxonomic loss affects functional diversity over short and long timeframes. In a macroevolutionary model system, we find that, despite losing ~60% of genera and ~20% of family-level diversity, marine bivalves lost only ~5% of their functional diversity, inconsistent with random extinction. Even with evolutionary opportunities presented by a disrupted ecosystem, low-diversity groups prior to the extinction or those originating in the Cenozoic rarely reach higher ranks today, implying long-term diversity ceilings to certain ecological roles. Clades that survived the extinction tend to dominate functions today, 66 million years post-extinction, but both relative richness and phylogenetic structure of those functional groups have been significantly shuffled. Thus, neither the composition of the pre-extinction biota nor the set of taxa that survived the extinction fully accounts for the functional and phylogenetic structure of today’s biota. The extinction disrupted Mesozoic biodiversity but did not fully determine the present-day configuration.
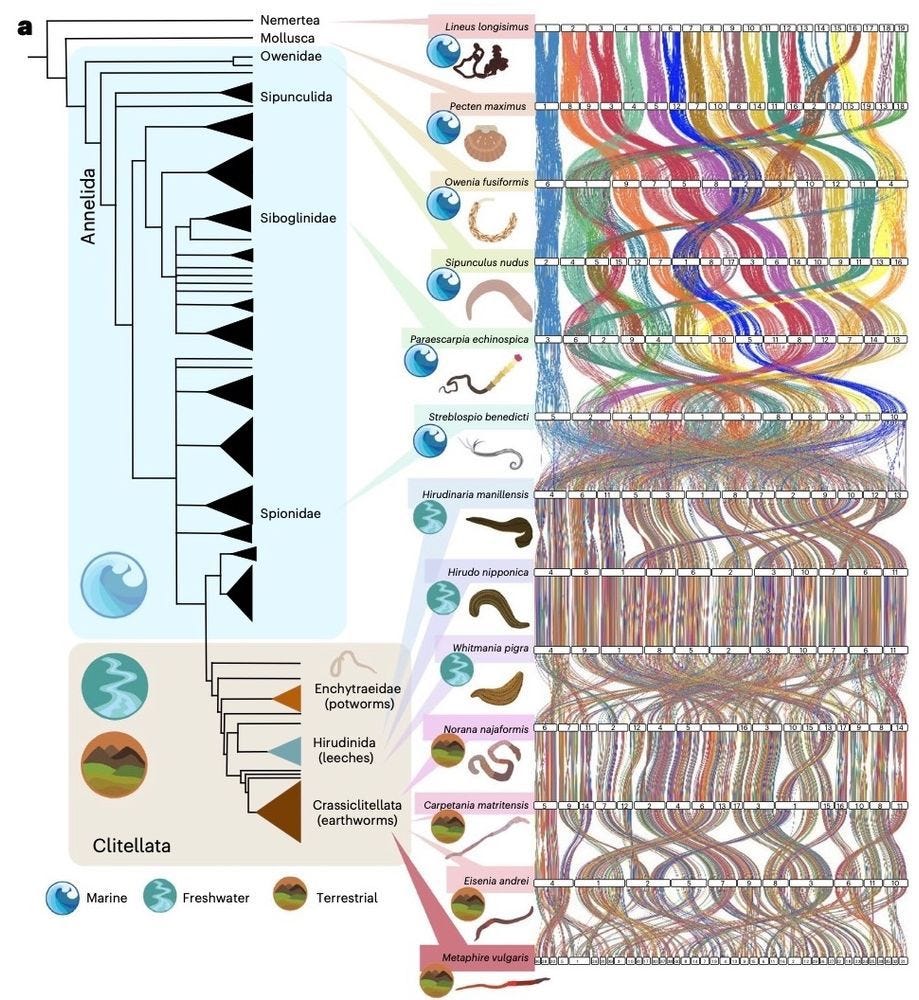
An episodic burst of massive genomic rearrangements and the origin of non-marine annelids
Every now and then, evolution shows us just how creative it can be. In the case of earthworms and their relatives, their shift from sea to land was sparked by a rare burst of genome-wide chaos, literally breaking, reshuffling and rewiring chromosomes in ways most species couldn’t tolerate [as far as we know]. That these organisms not only survived but thrived reminds us that life, as a complex adaptive system, can turn instability into opportunity, using even disruption as raw material for resilience and innovation.
The genomic basis of cladogenesis and adaptive evolutionary change has intrigued biologists for decades. Here we show that the tectonics of genome evolution in clitellates, a clade composed of most freshwater and all terrestrial species of the phylum Annelida, is characterized by extensive genome-wide scrambling that resulted in a massive loss of macrosynteny between marine annelids and clitellates. These massive rearrangements included the formation of putative neocentromeres with newly acquired transposable elements and preceded a further period of genome-wide reshaping events, potentially triggered by the loss of genes involved in genome stability and homoeostasis of cell division. Notably, whereas these rearrangements broke short-range interactions observed between Hox genes in marine annelids, they were reformed as long-range interactions in clitellates. Our findings reveal extensive genomic reshaping in clitellates at both the linear (2D) and three-dimensional (3D) levels, suggesting that unlike in other animal lineages where synteny conservation constrains structural evolution, clitellates exhibit a remarkable tolerance for chromosomal rearrangements. Our study thus suggests that the genomic landscape of Clitellata resulted from a rare burst of genomic changes that ended a long period of stability that persists across large phylogenetic distances.
Ecosystems
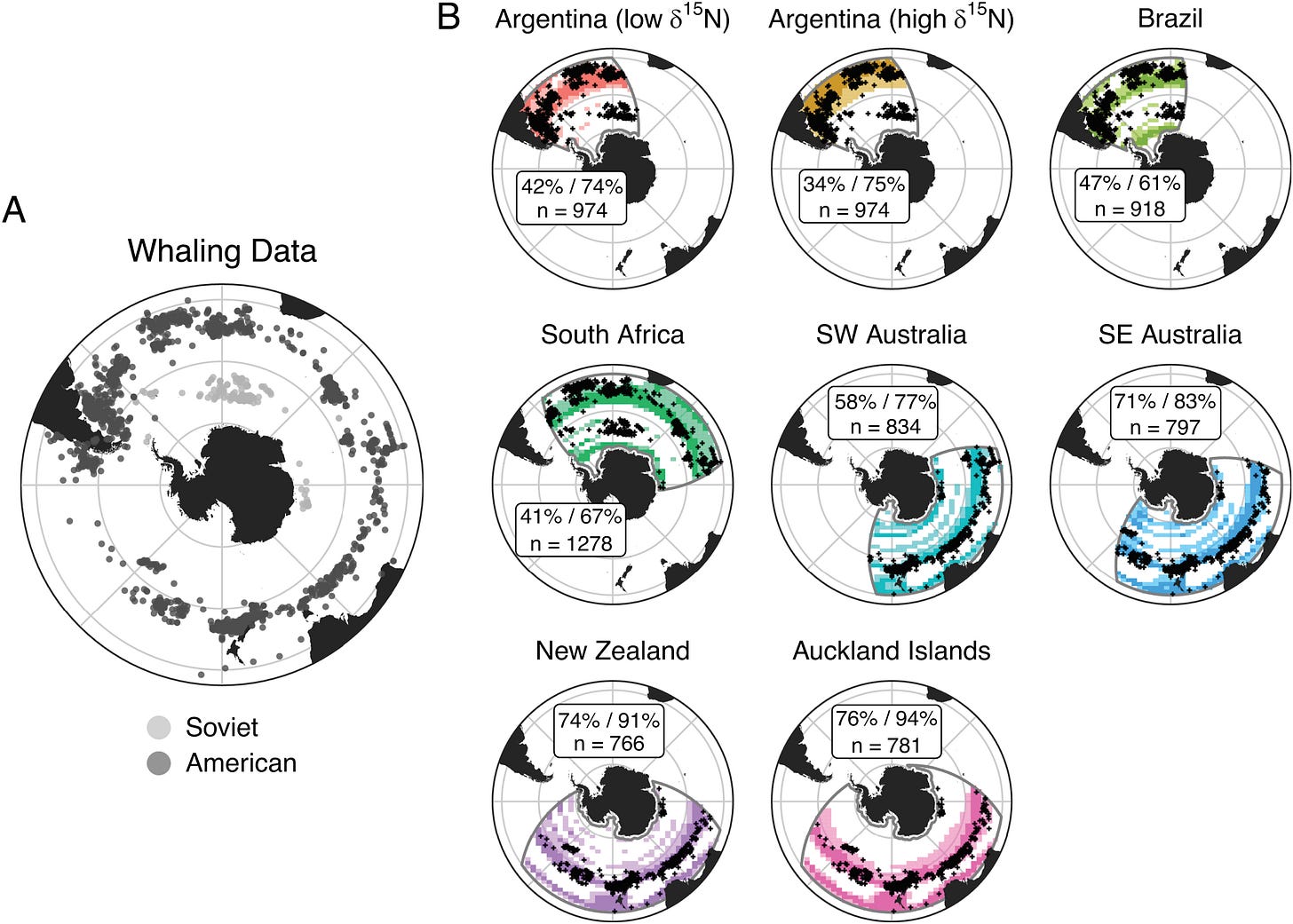
SRWs appear stable in their use of the mid-latitude foraging grounds, despite potential loss of cultural migratory memory after the whaling era
Assessing change in Southern Ocean ecosystems is challenging due to its remoteness. Large-scale datasets that allow comparison between present-day conditions and those prior to large-scale ecosystem disturbances caused by humans (e.g., fishing/whaling) are rare. We infer the contemporary offshore foraging distribution of a marine predator, southern right whales (n = 1,002), using a customized stable isotope-based assignment approach based on biogeochemical models of the Southern Ocean. We then compare the contemporary distributions during the late austral summer and autumn to whaling catch data representing historical distributions during the same seasons. We show remarkable consistency of mid-latitude distribution across four centuries but shifts in foraging grounds in the past 30 y, particularly in the high latitudes that are likely driven by climate-associated alterations in prey availability.
Assessing environmental changes in Southern Ocean ecosystems is difficult due to its remoteness and data sparsity. Monitoring marine predators that respond rapidly to environmental variation may enable us to track anthropogenic effects on ecosystems. Yet, many long-term datasets of marine predators are incomplete because they are spatially constrained and/or track ecosystems already modified by industrial fishing and whaling in the latter half of the 20th century. Here, we assess the contemporary offshore distribution of a wide-ranging marine predator, the southern right whale (SRW, Eubalaena australis), that forages on copepods and krill from ~30°S to the Antarctic ice edge (>60°S). We analyzed carbon and nitrogen isotope values of 1,002 skin samples from six genetically distinct SRW populations using a customized assignment approach that accounts for temporal and spatial variation in the Southern Ocean phytoplankton isoscape. Over the past three decades, SRWs increased their use of mid-latitude foraging grounds in the south Atlantic and southwest (SW) Indian oceans in the late austral summer and autumn and slightly increased their use of high-latitude (>60°S) foraging grounds in the SW Pacific, coincident with observed changes in prey distribution and abundance on a circumpolar scale. Comparing foraging assignments with whaling records since the 18th century showed remarkable stability in use of mid-latitude foraging areas. We attribute this consistency across four centuries to the physical stability of ocean fronts and resulting productivity in mid-latitude ecosystems of the Southern Ocean compared with polar regions that may be more influenced by recent climate change.
Biological Systems
Should I stay or should I go: transmission trade-offs in phages and plasmids
After three years working with lab on the dynamics of microbial communities, I started to imagine bacteria as tiny traders in a bustling marketplace, constantly exchanging goods. One of their hottest commodities is the plasmid: a portable DNA package that can carry powerful survival tools, like antibiotic resistance genes. Yes, in practice plasmids can provide bacteria with superpowers.
Unlike inheritance in humans, where traits pass only from parent to child, plasmids can leap sideways between unrelated bacteria, spreading resistance through entire microbial neighborhoods in record time. This genetic trade does not appear to be random: it speeds up when bacteria are crowded, stressed or competing with other mobile DNA elements. By studying how and when plasmids move, we can uncover the hidden rules of this microbial economy, knowledge that’s vital for keeping under control the rise of antibiotic-resistant “superbugs” that threaten modern medicine.
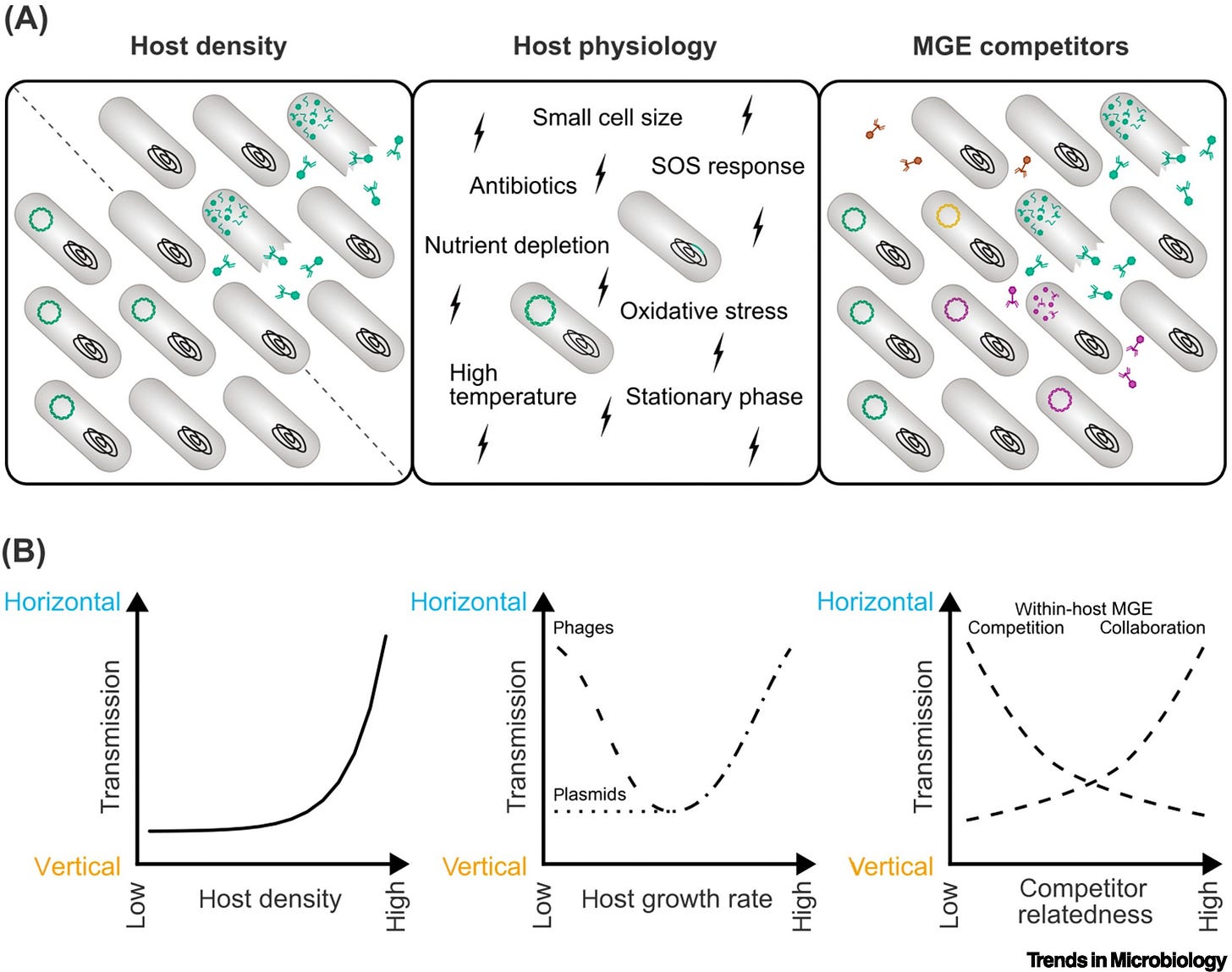
Temperate phages and conjugative plasmids need to balance horizontal and vertical transmission for their reproductive success. The optimal balance depends on the environment. These mobile genetic elements (MGEs) have different lifestyles yet remarkably similar transmission trade-offs. Selection resulting from such trade-offs shapes the regulation of MGE transmission.
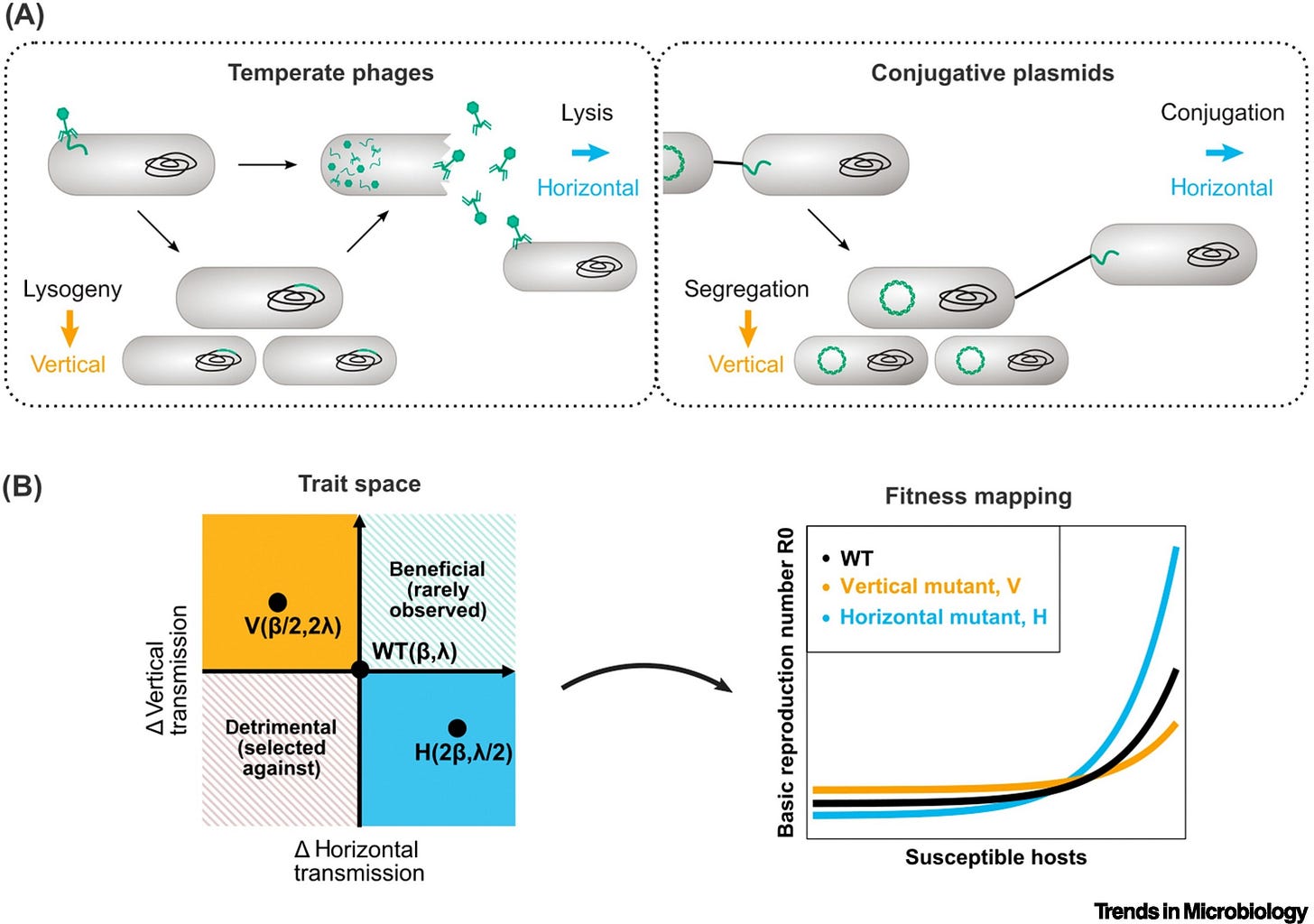
Both conjugative plasmids and temperate phages can regulate their transmission in response to host cell density. Differences between the two elements are found in the response to host metabolism, stressors and competing MGEs. Existing theory does not capture all observed behavior and new, MGE-specific predictions are needed.
The fields of phage and plasmid biology can help each other identify gaps in the literature, such as shared regulatory cues, and reveal general rules of transmission regulation in MGEs.
Mobile genetic elements (MGEs), like temperate bacteriophages and conjugative plasmids, are major vectors of virulence and antibiotic resistance in bacterial populations. For reproductive success, MGEs must balance horizontal and vertical transmission. Yet, the cost of horizontal transmission (metabolic burden or host death) puts these transmission modes at odds. Using virulence–transmission trade-off (VTT) theory, we identify three groups of environmental variables affecting the balance between horizontal and vertical transmission: host density, host physiology, and competitors. We find that general theoretical predictions of the optimal response to environmental cues align with experimental evidence on the regulation of transmission by phages and plasmids. We further highlight gaps between theory and experiments, differences between phages and plasmids, and suggest areas for future research.
→ Please, remind that if you find value in #ComplexityThoughts, you might consider helping it grow by subscribing, or by sharing it with friends, colleagues or on social media. See also this post to learn more about this space.